1. TeCl2 Lewis Dot Structure - Polar or Nonpolar, Bond Angle, Molecular ...
This video shows you how to draw the lewis dot structure of TeCl2 also known as tellurium dichloride. It discusses if TeCl2 is polar or nonpolar in addition ...
This video shows you how to draw the lewis dot structure of TeCl2 also known as tellurium dichloride. It discusses if TeCl2 is polar or nonpolar in addition to the bond angle, hybridization, and the molecular geometry.

2. TELLURIUM DICHLORIDE | 10025-71-5 - ChemicalBook
Oct 17, 2023 · Chemical Name: TELLURIUM DICHLORIDE ; CBNumber: CB7373583 ; Molecular Formula: Cl2Te. Lewis structure ; Molecular Weight: 198.51 ; MDL Number: ...
Visit ChemicalBook To find more TELLURIUM DICHLORIDE(10025-71-5) information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes. You can also browse global suppliers,vendor,prices,Price,manufacturers of TELLURIUM DICHLORIDE(10025-71-5). At last,TELLURIUM DICHLORIDE(10025-71-5) safety, risk, hazard and MSDS, CAS,cas number,Use,cas no may also be you need.
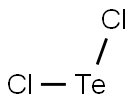
3. BeCl2 and TeCl2 are both covalent molecules, yet BeCl2 is linear while ...
Dec 23, 2015 · TeCl2 : The central Te atom has 6 valence electrons. When the two Cl atoms bond to the central atom, they each contribute one electron to ...
"TeCl"_2: The central "Te" atom has 6 valence electrons. When the two "Cl" atoms bond to the central atom, they each contribute one electron to form two sigma bonds. This leaves 4 electrons on the central atoms in the form of 2 lone electron pairs. This is the VSEPR shape "AX"_2"E"_2, a tetrahedron shape with two electron pairs and two atoms, creating a bent molecular shape. "BeCl"_2, on the other hand, has "Be" as its central atom. Since "Be" only has 2 valence electrons, the two sigma bonds formed by the "Cl" atoms leave "Be" with no lone pairs. The VSEPR shape is "AX"_2"E"_0 and forms a linear shape.

4. Draw the Lewis structures for TeCl2, ICl, PCl3, KrCl2, and XeCl4. Which ...
Nov 4, 2021 · Draw the Lewis structures for TeCl2, ICl, PCl3, KrCl2, and XeCl4. Which of the compounds exhibit at least one bond angle that is approximately ...
VIDEO ANSWER: Four bands. There are electron particles. It is on this. Six months is a long time. Seven to four. 33. The crack we sell is from the Louis struct…
5. 1. Help me understand how to write the correct format for the Lewis ...
Duration: 1:34Posted: Mar 14, 2024
VIDEO ANSWER: Hello students were given by ocelli, which cato the details about the covalent bonds to covalent bonds are formed by sharing of electron taring o…
6. Draw Lewis dot structures for the molecules listed here. a. TeCl2 b ...
Jun 25, 2019 · VIDEO ANSWER: Okay. Lewis dot structure for Fleury. Um, die chloride. Well killed a total number of valence electrons.
VIDEO ANSWER: Okay. Lewis dot structure for Fleury. Um, die chloride. Well killed a total number of valence electrons. Uh, delirium have six. Then we've got se…
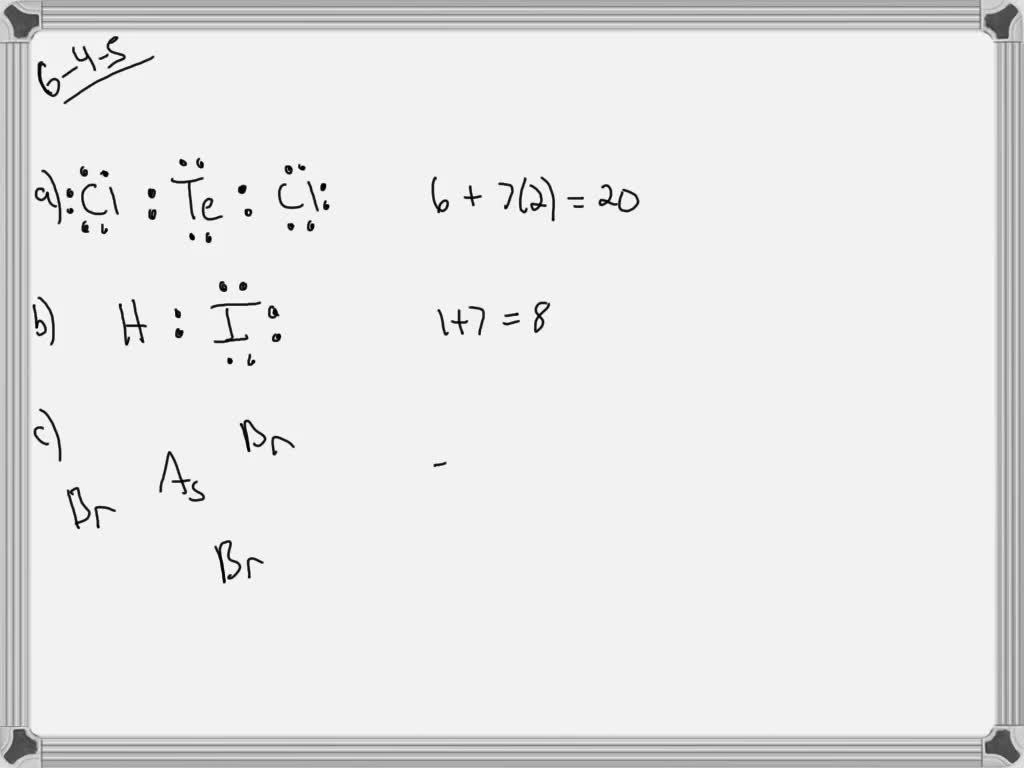
7. WHAT IS TeCl2? - Answers
Dec 7, 2007 · What is the molecular shape of TeCl2? ... When Ge binds to the two Cl, 2 single (sigma) bonds are created, resulting in a Lewis structure with: ..
Tellurium Chloride

8. Draw the Lewis structures for TeCl2, ICl, PCl3, KrCl2, and XeCl4. Which ...
Duration: 1:34Posted: Dec 9, 2021
VIDEO ANSWER: the T E C l four number of bands. Electrons. On this. It's six months. Seven into four. 34. This is what we sell crack from when we do the Louis …
9. Molecular Structure and Polarity | Chemistry for Majors
This molecule has regions of high electron density that consist of two single bonds and one double bond. The basic geometry is trigonal planar with 120° bond ...
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 1). A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance (or bond length) is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Bond distances are measured in Ångstroms (1 Å = 10–10 m) or picometers (1 pm = 10–12 m, 100 pm = 1 Å).
10. Starting from the Lewis structure, determine the hybridization type of ...
Duration: 1:34Posted: Mar 22, 2022
VIDEO ANSWER:
11. AP Chem =( - AP Test Preparation - College Confidential Forums
Dec 20, 2005 · ... How can you tell if compounds are polar ??? For example…
TeCl2 PH3 AsCl5 RnF4 IBr4 -1 ?? Thanks ...
Draw out the Lewis Structures for ...
How can you tell if compounds are polar ??? For example…
TeCl2 PH3 AsCl5 RnF4 IBr4 -1 ?? Thanks

12. [PDF] Terminating species and Lewis acid-base preference in oxohalides
Lewis acids to strong Lewis bases and weak acids to weak bases. ... (Fe(CO)3)2Cl(TeCl2)2. (Te2Cl10). J.R. Eveland et al ... the same position in a structure if they ...